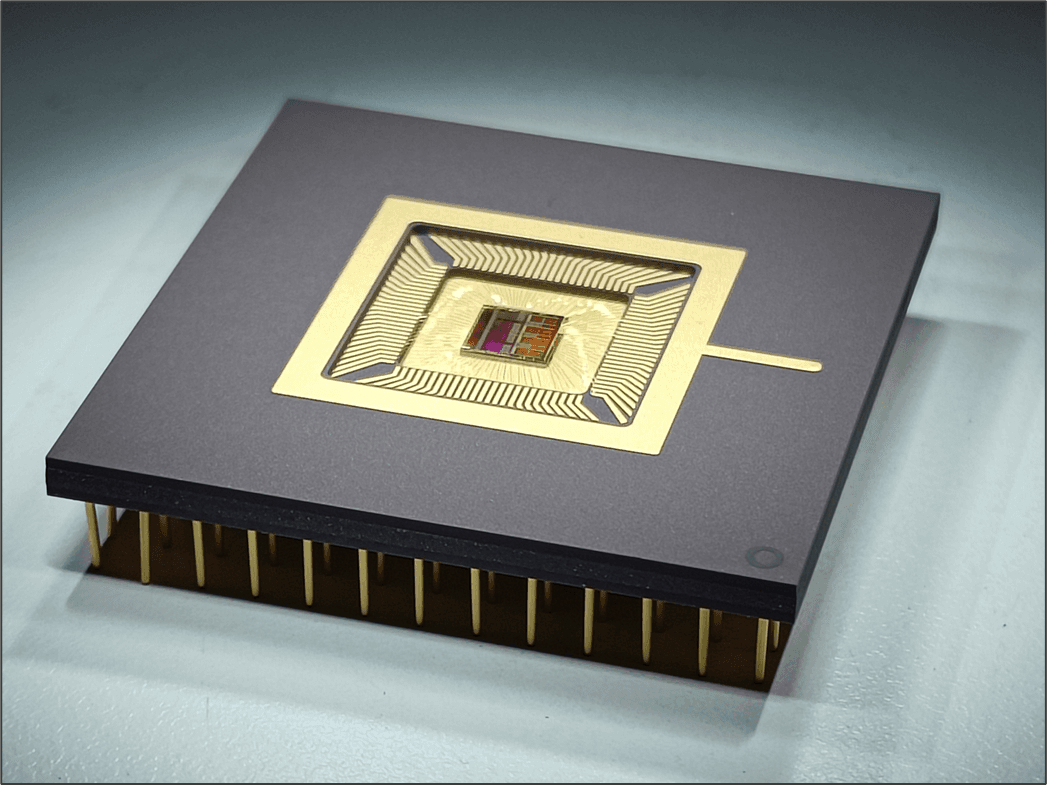
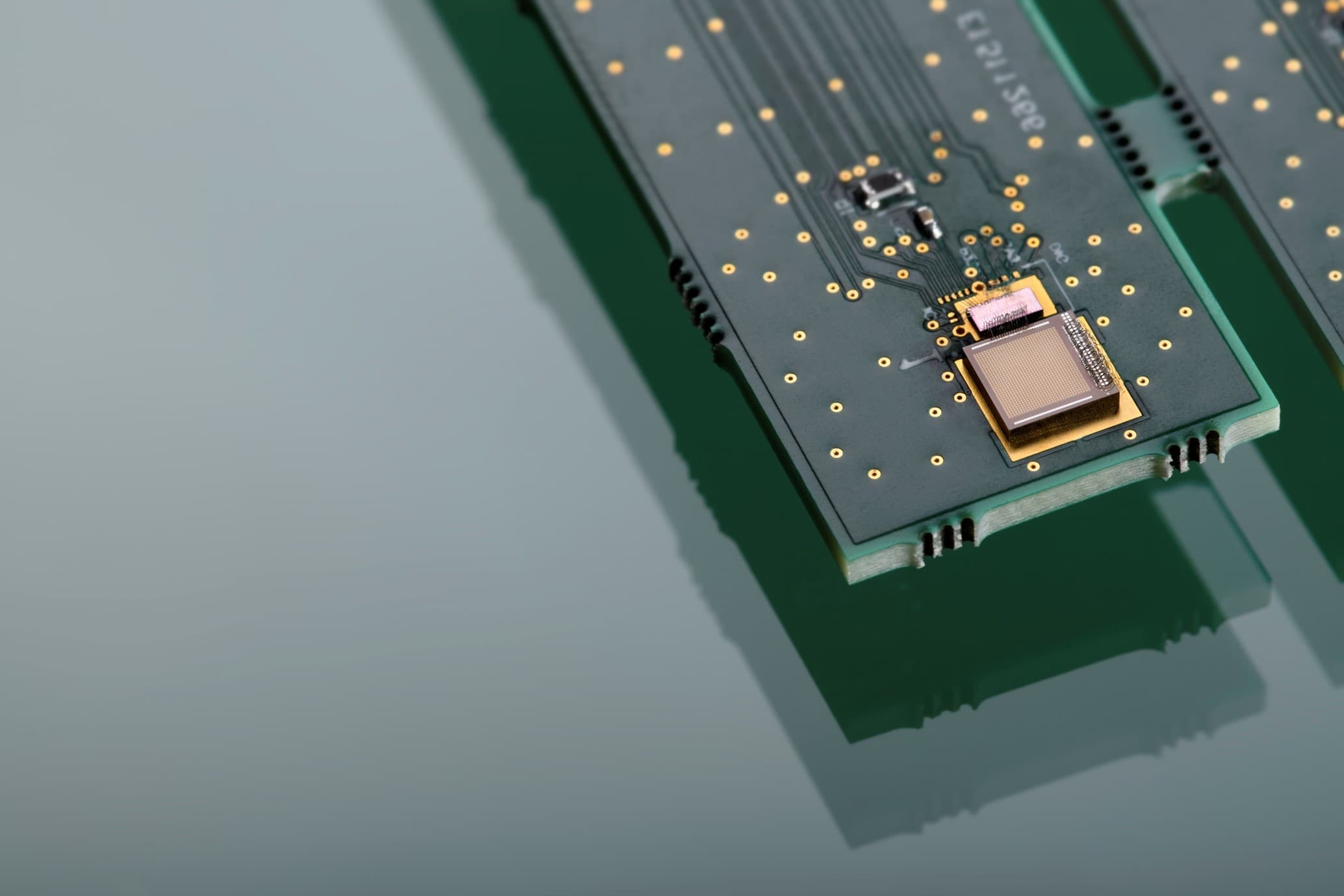
At ISSCC 2020 (San Francisco), imec presented the world’s first millimeter-scale wireless transceiver for ingestibles, or electronic pills. Nick Van Helleputte, R&D manager connected health solutions at imec and Chris Van Hoof, vice-president connected health solutions at imec and general manager of the OnePlanet Research Center in Gelderland (NL), shed their light on the specific challenges they face in the emerging medical research on ingestibles and how this translates into imec’s R&D roadmaps.
Seventy years and still (hardly) no ingestibles
Electronic analytical devices that need to be swallowed are not new. Wikipedia reports the first such device already existed in the mid 20th Century. Yet, to date, apart from camera pills that can be swallowed, ingestibles remain very much an explorative field of R&D with little or no widely accepted applications. Whenever the need to investigate or intervene in our digestive tract, medical practitioners still have to rely on relatively invasive and cumbersome tools such as endoscopes. Having an additional drawback of being momentary observations, a shortcoming they share with for example sample based stool analyses.
Wondering why this ‘seventy-year old’ technology domain of ingestibles hasn’t seen more substantial application breakthroughs yet, two main reasons can be identified: the generic electronic challenges (such as miniaturization) and the more application-oriented challenges (such as what to measure and how).
Electronics are (not) the problem
Looking at the mere electronics’ challenges, one could argue there are little or no fundamental showstoppers to be expected on the horizon. The requirements on materials usage for biocompatibility being even somewhat more relaxed as for implants, since ingestibles pass through our digestive tract and therefore in essence do not enter our body.
Yet, the seventy years that have passed since the first swallowable electronics device have been needed to even allow for miniaturized technologies to be available. And also more specific technologies such as microfluidics and bio-electronics are only going through accelerated developments in the last decades.
While the micro- and nanoelectronics roadmaps for ingestibles contain the usual ingredients such as miniaturized form factor, reliable wireless communication and ultra-low power consumption; substantial improvements are still needed compared to the current state-of-the-art. Imec’s recent breakthrough in a dedicated radio chip for ingestibles being a tangible proof of recent progress. So, although there are no immediate showstoppers to be expected, there certainly still is a lot of effort waiting ahead.
If one electronics development needs to be named as critical for further development, it’s the one of transducers. Or more specifically: codesigning novel transducers with optimized readout electronics.
Needed to translate the sensory input to a useful readout signal that can be effectively amplified and digitized, with as little energy needed as possible. For wearables this usually means converting an electrophysiological signal, such as ECG or EEG, to a digital output: so electrical to electrical. In contrast, ingestibles face a myriad of input signals, which can be biological, chemical, physical… Translating these into a useful output signal leaves system developers with multiple options: optical, impedance, electrical, mechanical…
Which leads to the real challenge in ingestibles R&D, which is not about the electronics components per sé, but about making the right choices in what to measure and how.
Chicken and egg: exploration of a 10-meter long living tunnel
Fluid and electrolyte balance, metabolites (glucose, lactate, short-chain fatty acids…), hormones and neuromodulators, bacteria (pathogenic or others)… the list of potentially interesting biochemical targets is endless. Conventional analytics methodologies, such as blood, stool and urine analyses, are powerful enough to give an almost complete composition and to research a multitude of parameters in one sample. They don’t even require to know upfront what exactly you are looking for.
Not so for ingestibles.
Due to the system constraints, extreme focus is needed.
For each target parameter, a dedicated and specific sensor needs to be developed, including its one-component transducer. Which at the moment results in sort of a chicken and egg situation: more medical and biological knowledge is needed to make the right technology choices and more data from technology-based research is needed to drive the medical and biological knowledge. For example, if we know parameter X leads to clinical effect Y, how can we translate its critical thresholds that are known for blood or stool samples to measurements taken directly from within the digestive tract?
The synergetic effect of technological and clinical research is a determining factor for the progress in the domain. An explosion of technical research papers on ingestibles can be observed over the last five years; mostly in the domains of transducers and analytes. And, not coincidentally, around a year later followed by clinical publications reporting on the medical and biological insights obtained with these newest technologies.
Aside from this bio-tech interplay, the digestive tract is far from a homogeneous and static environment. Throughout the more or less ten meters, the environment – containing liquids, solids and gasses – does not only change over distance (throat, stomach, gut…), but also over time (e.g. empty or full stomach) ánd over location (a lot of biochemical reactions happen at the intestinal walls). So other than let’s say air quality measurements in traffic tunnels, contextual knowledge on where the sensor is located and at what time is essential.
Imec’s choice of ingredients for the technology roadmap
In terms of technology choices for sensing, there’s only so much one can detect with a camera. Even if indirectly visual (or other) light detection is also being used in bioluminescence-based systems. Literature reports groups who for example use genetically modified bacteria as bio-labels: these light up when binding to the desired metabolite, allowing for detection by a pill with a suitable optical sensor.
In contrast, imec’s roadmaps mostly focus on label-free sensing methods. Various electro-chemical sensing methods are realistic options for implementation in ingestibles. These typically prove to be somewhat less sensitive as label-based detection, yet for most applications are more than sufficient. On the upside, they are less complicated – as they require no additional interventions such as introducing the label – and therefore easier to implement within the ingestibles’ system constraints.
Another aspect is the location detection and control. Whereby the location detection itself starts with the right frame of reference.
Unlike what some might think, the location of the pill in an external reference frame – like with gps – is not relevant. Rather, you want to know how far in the digestive tract the pill is.
Has it gone through X or Y bends in the gut? Etc. This type of location detection asks for a specific approach, for which we are only in the exploratory phases of research. A possible approach is to make use of mainstream components such as accelerometers and gyroscopes, which are available anyhow in the sensor’s inertial measurement unit (IMU).
In terms of location control, there is a choice between free-floating systems or more controlled ones. Free floating systems move forward through the inherent functioning of our digestive tract. The primary technological challenge is to provide all of the above functionality in a small enough device so there is no risk for obstruction, retention or blockage.
Using free-floating systems, you have no control over the location or transit time. One could imagine systems whereby the movement and actuation happen in a more controlled way. For example by wireless impulses from an external device triggering a capillary system in the ingestible to take a sample or perform an analysis at a certain moment or location. Or pills that have specific coatings that can be dissolved automatically (e.g. at a certain pH) or actively (e.g. via an electrical signal) in order to activate certain functions at the right time or location. All options we are actively researching at imec. And the communication with the outside world that is needed to enable this, has now been made possible by our recent breakthrough wireless transceiver.
Something we are not actively pursuing is steering of ingestibles through e.g. microbots or -propellers, as is being reported in literature. Rather, we opt for systems that can attach themselves at a certain location if needed. For example if certain biomarkers are not sufficiently present in the inner stomach and need to be measured directly against the wall. Or if you want to measure at a certain location throughout the whole digestive cycle.
For such purposes, one could think of ingestibles with tiny needles that get activated – through dissolvable coatings or an external trigger – once they arrive at the right location. Pinning down the ingestible until, after a certain time, they are being organically dissolved or again triggered to release by an external signal.
Imec’s application roadmap targets three domains
But what is to be expected on the application horizon? Aside from the already mentioned camera pills, which have been commercially available for over a decade, variants exist with features such as pH, temperature and pressure measurements. These devices have a clinical use, but are mostly limited to visual observations and transit-time analysis. Also, some pills measure core temperature: a useful parameter for top athletes to monitor the intensity of their efforts, yet commercially rather a niche market. Another niche is pills that have some sort of mechanism that get’s triggered when they are taken in order to monitor – and increase – the patient’s compliance to his or her prescription.
Slowly but surely we observe an interest from industry for also other applications and use cases. So we are taking these existing ideas and bring them further by providing much more capable sensing and eventually also stimulation/drug delivery.
At imec, we target three main application areas: gut health, early biomarkers and nutrition.
Gut health being all that has to do with your digestion directly (indigestion, transit problems, leaky gut…). Detection of early biomarkers as diagnostic (or therapeutic) support for diseases such as diabetes, Crohn’s, coeliac… And nutrition through for example monitoring of vitamins or other analytes in our overall pursuit for wellness and healthy living.
Within each of these domains, clinical wish lists exist with potential analytical targets that could lead to progressive insights. This helps to narrow down the options in the technology roadmaps. Also, in a first instance, imec starts by developing advanced clinical research tools before aiming for ingestibles for dedicated applications. For example, one could think of first introducing sampling pills that collect and label a multitude of samples for analysis with conventional lab tools. After which the insights from these samples can help determine the further development focus towards specific end products.
Overall, imec aims to get an holistic view on the future developments. Resulting in the capability to measure an ever-growing list of parameters in a more elaborate and continuous way. With imec’s technology portfolio, encompassing all relevant aspects for miniaturization, powerful sensing and communication, microfluidics, biochemistry etc, as a unique toolset to help and bring light at the end of the tunnel.
More information
-
Read more about our expertise in ingestible sensors here.

Nick Van Helleputte received his MSc degree in electrical engineering in 2004 from the Katholieke Universiteit Leuven, Belgium. He received his PhD from the same university in 2009 (KU Leuven MICAS research group). His PhD research focused on low-power ultra-wide-band analog front-end receivers for a wide range of applications. He joined imec in 2009 as an Analog R&D Design Engineer. He is currently R&D manager of the connected health solutions team. His research focus is on ultra-low-power circuits for biomedical applications. He has been involved in analog and mixed-signal ASIC design for wearable and implantable healthcare solutions. Nick is an IEEE member and served on the technical program committee of VLSI circuits symposium and ISSCC.

Making a difference in patient’s lives and effectively contributing to the challenge of disease prevention are the true motivators behind the innovations and HW/SW solutions which Chris is creating at imec. After receiving a PhD in Electrical Engineering from the University of Leuven in 1992, Chris has held positions as manager and director at imec in highly diverse fields spanning technology, circuits, systems, data and applications. Chris is currently vice-president of the Connected Health Solutions R&D and general manager of the OnePlanet Research Center in Gelderland. Apart from delivering industry-relevant qualified solutions to customers, his work also resulted in five startups (four in the healthcare domain). He is also full professor at the University of Leuven and imec Fellow.
Published on:
13 February 2020