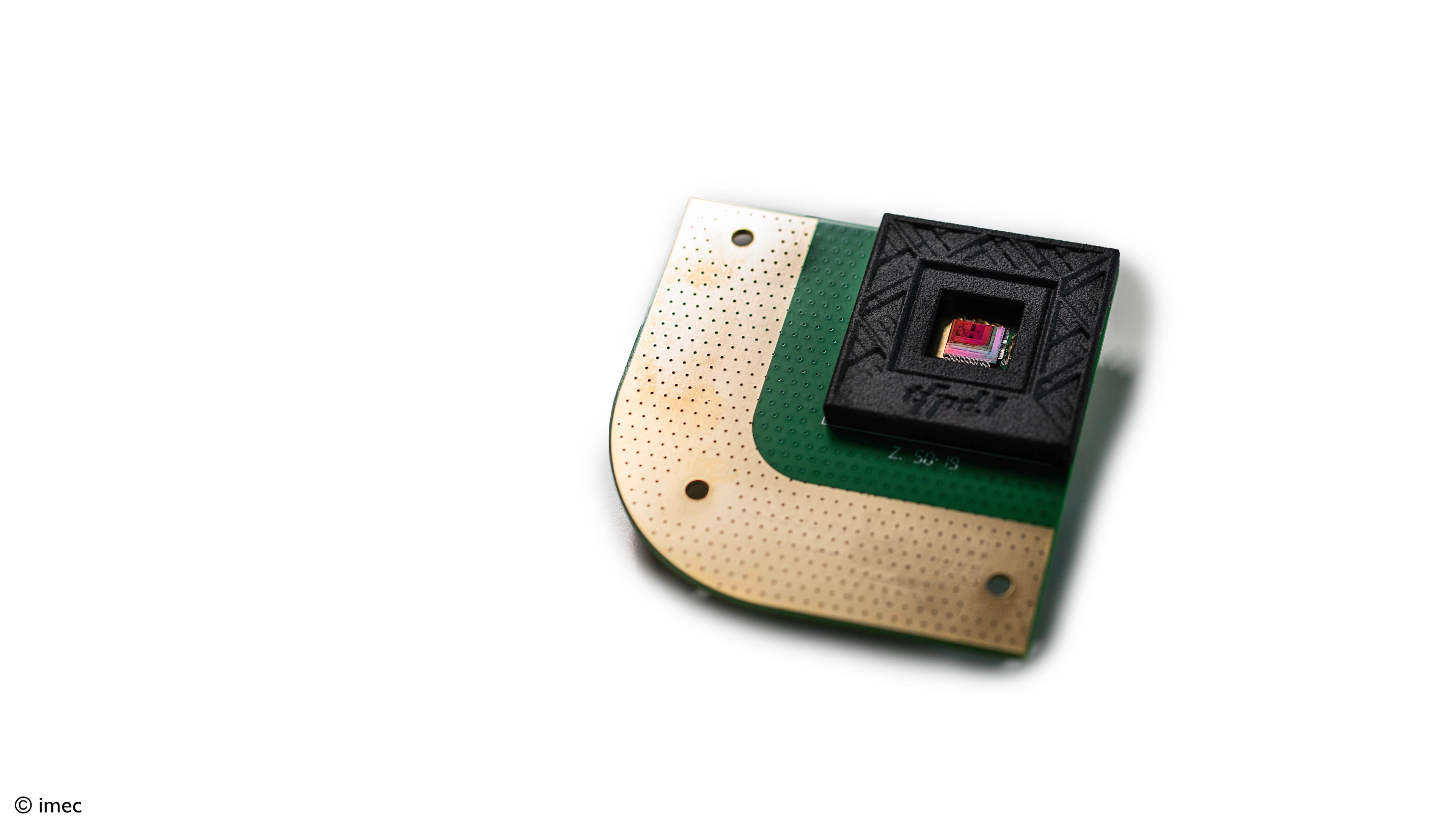
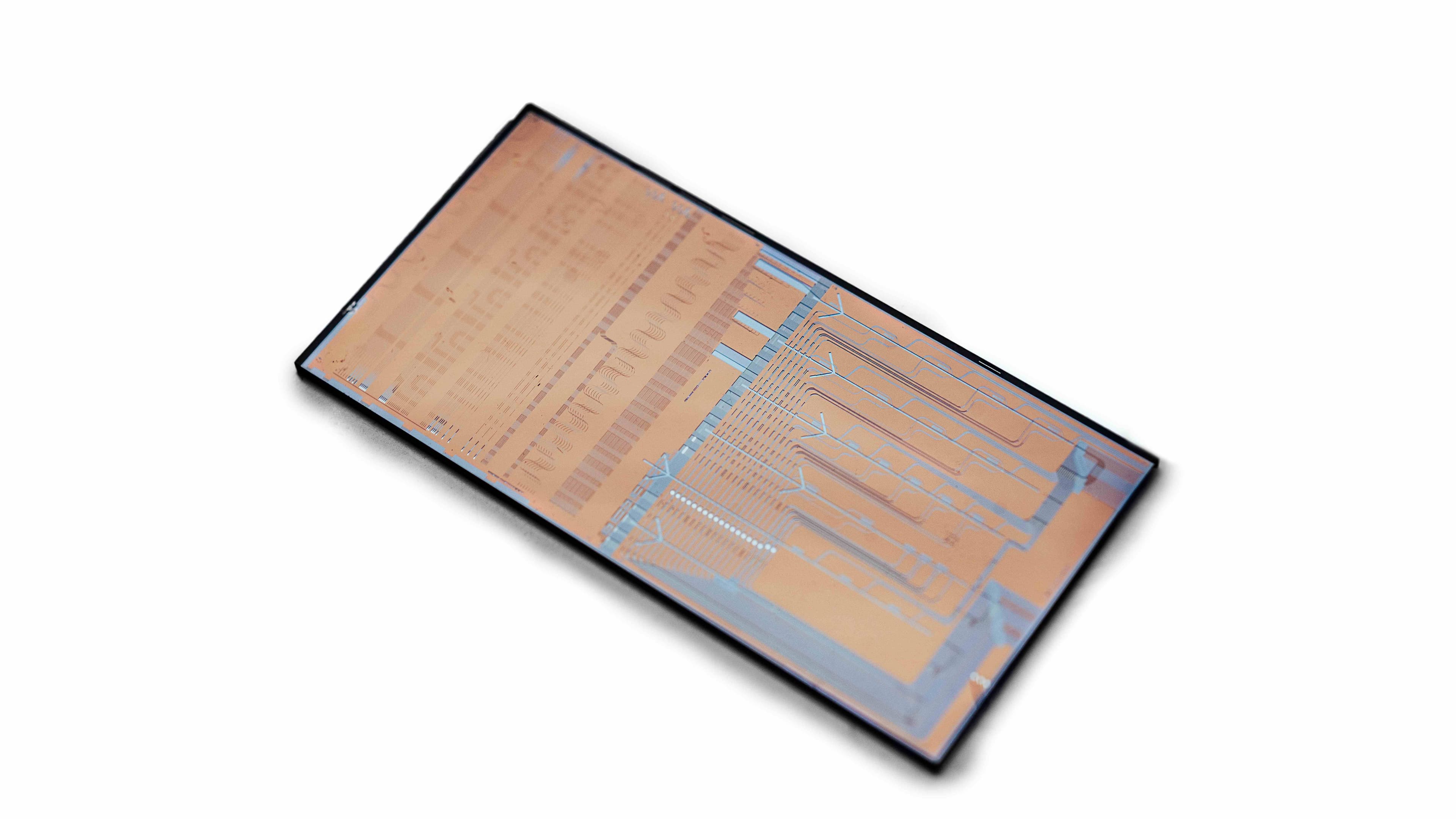
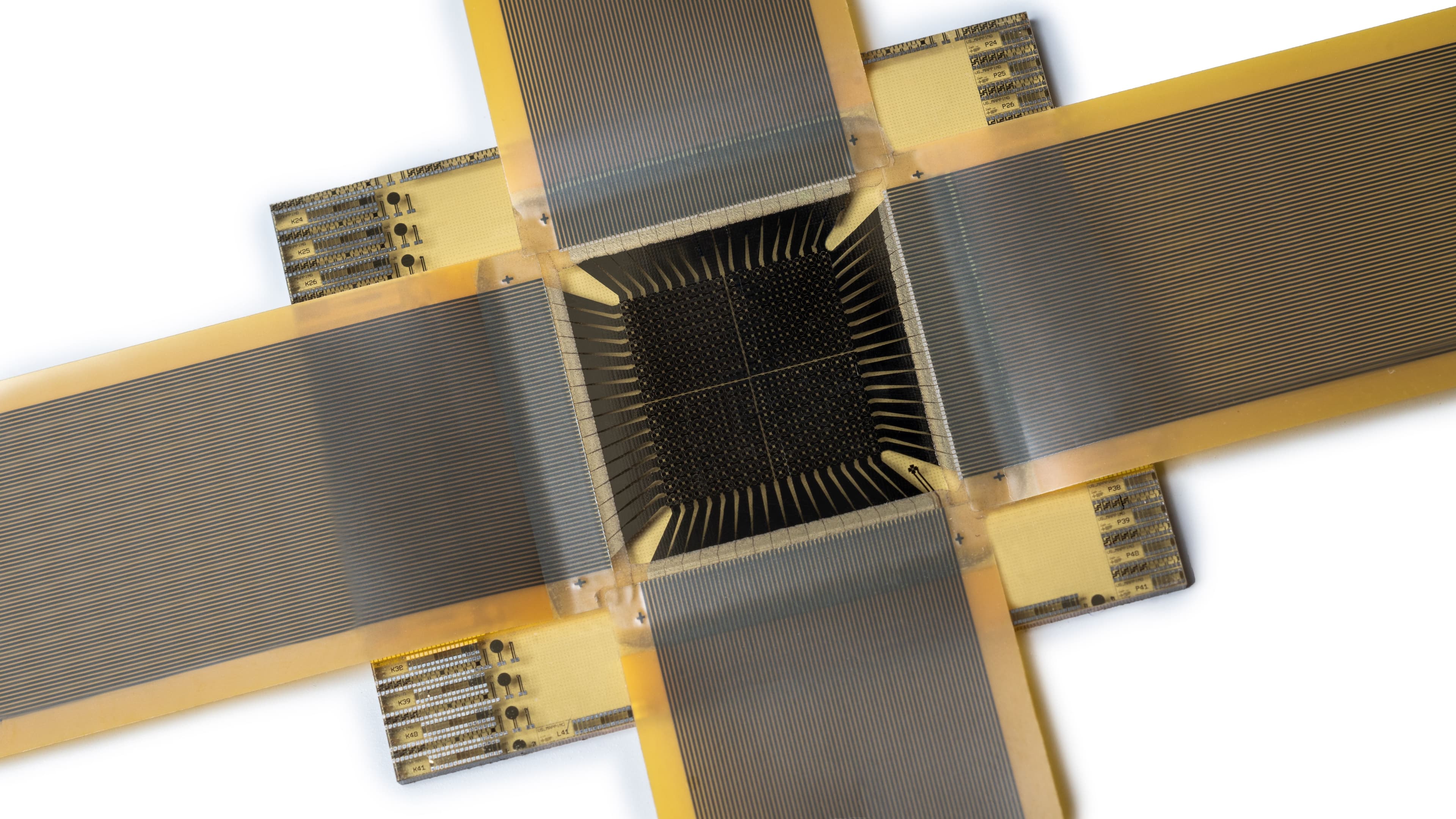
Breath sampler
Breathe and wait no more than 20 minutes for an accurate result on your biomarkers. That’s the experience that imec aims for with its breath-based testing tool.
Breath analysis technology can detect a variety of diseases and medical conditions ranging from respiratory diseases to metabolic and infectious diseases (e.g. bacterial infections such as Tuberculosis, viral infections such as COVID-19, and potentially COPD exacerbations).
The problem with most of the tests for infectious diseases or respirairatory diseases is that the sample collection is often uncomfortable for the patient, and that the analysis takes a lot of time; time that is precious for the patient and – in case of contagious diseases – for his environment. Other drawbacks are reliability, complexity, and precision.
Imec strongly believes in the power of aerosols for diagnostics and for gaining insights in disease transmissions. That's why imec developed a new breath-test prototype that is not based on exhaled volatile organic compounds (VOCs) but on RT-qPCR, leveraging imec’s existing rapid on-chip PCR technology building blocks (time to result can be reduced up to 5’). This last technique, used in clinical laboratories, is known for its high sensitivity and specificity. With its breath sampler, imec aims to introduce fast, easy and accurate identification of disease markers in breath.
Work with us
Use cases
A breath-based testing device that's derived from imec's breath sampler concept will be:
- easy and comfortable to use
- sensitive, even in the pre-symptomatic stage (in case of infectious diseases)
- fast, with test results within 18 minutes
That makes it ideal for use in airports, at a hospital’s emergency unit or a GP’s office.
Test results within < 20 minutes
Imec leverages silicon chip technology to develop a new testing approach. We’re working on a breath sampler that e.g. detects COVID-19 viral particles that are contained in aerosols and droplets.
Watch the video to see how the new test combines the ease of a breath sampler with the sensitivity of a PCR analysis.
Features:
- easy sampling procedure
- quantifiable virus collection & sample collection method
- high sensitivity and specificity, due to use of the PCR testing method
- rapid process: 1 minute for the sampling, and about 18 minutes for the analysis
- versatile platform: adaptable to various respiratory pathogens
Because this solution is based on standard chip technology, it’s mass-producible. It can be quickly made accessible in unlimited amounts (e.g. in case of a pandemic that spreads via exhaled particles).
Imec performed several clinical studies with its breath sampler. Below is one of the results that was achieved in such a study.

Viral load as a function of day since infection as measured using an RT-qPCR on a nasopharyngeal swab sample (grey) and breath (cyan) for a cohort of 13 subjects. From day 9 on, people test negative with a breath-based test while still testing positive with a nasopharyngeal swab-based PCR test. Data for contemporaneous rapid antigen tests are shown below the graph with the green color representing the patient group that tests positive for SARS-CoV-2, red the negative-test group and white the group with missing results. The pie charts show that the rapid antigen tests in many cases give a negative result at the beginning of the infection while people are already infectious and can spread the disease. The new breath-based PCR test is as easy and fast as the antigen test but is much more accurate, especially during the first days of infection.
Want to get involved in this project? Feel free reach out.