Looking inside the body
Medical imaging techniques provide a unique view inside the body and are invaluable for diagnosis and disease monitoring. From X-ray, over MRI to ultrasound, the field is vast and diverse. When imaging biological tissue, the choice of the modality depends on the contrast used for imaging and the trade-off between resolution and depth. Light waves, e.g. used in endoscopy or microscopy, can generate high-resolution images but don’t travel far unperturbed. Deeper in tissue, the light gets scattered resulting in blurry images. High-energy X-rays form a special case as they penetrate deep into the tissue and yield high-resolution images, but this ionizing radiation limits their use.
To circumvent these drawbacks, other options that don’t rely on unperturbed light propagation have been explored as well. Acoustic or sound waves are well known to safely monitor foetuses in the womb using ultrasound imaging. These mechanical waves are less scattered than electromagnetic waves of comparable frequencies or wavelengths so they can reach objects deeper in the tissue. However, ultrasound images typically suffer from a low resolution. MRI (magnetic resonance imaging), based on radio waves that interact with hydrogen nuclei, shows similar features with a good depth exploration but limited resolution. MRI-images have greater detail than ultrasound images but they are typically not real-time and static. Moreover, MRI is a cumbersome technique that often requires contrast agents to enhance resolution.
In the soft spot between these established imaging methods emerges a novel technique with the resolution of light-based imaging and the good depth penetration of sound-based imaging, called photoacoustics (PA). It is capable of imaging finer blood vessels than other techniques without the need for contrast agents or X-ray exposure (photoacoustic imaging, PAI). Photoacoustics can also be applied for spectroscopy that describes the spectral characteristics of an object when light interacts with it (photoacoustic spectroscopy, PAS), for example, to identify biomolecules and monitor their concentrations based on a unique spectral signature (Figure 1). Imec is currently working on the technology to unlock the full potential of photoacoustics for biomedical applications. Hilde Jans, senior scientist and project manager for the photoacoustics activities in imec, and Xavier Rottenberg, fellow wave-based sensors and actuators at imec, discuss how semiconductor technologies can propel PA forward.
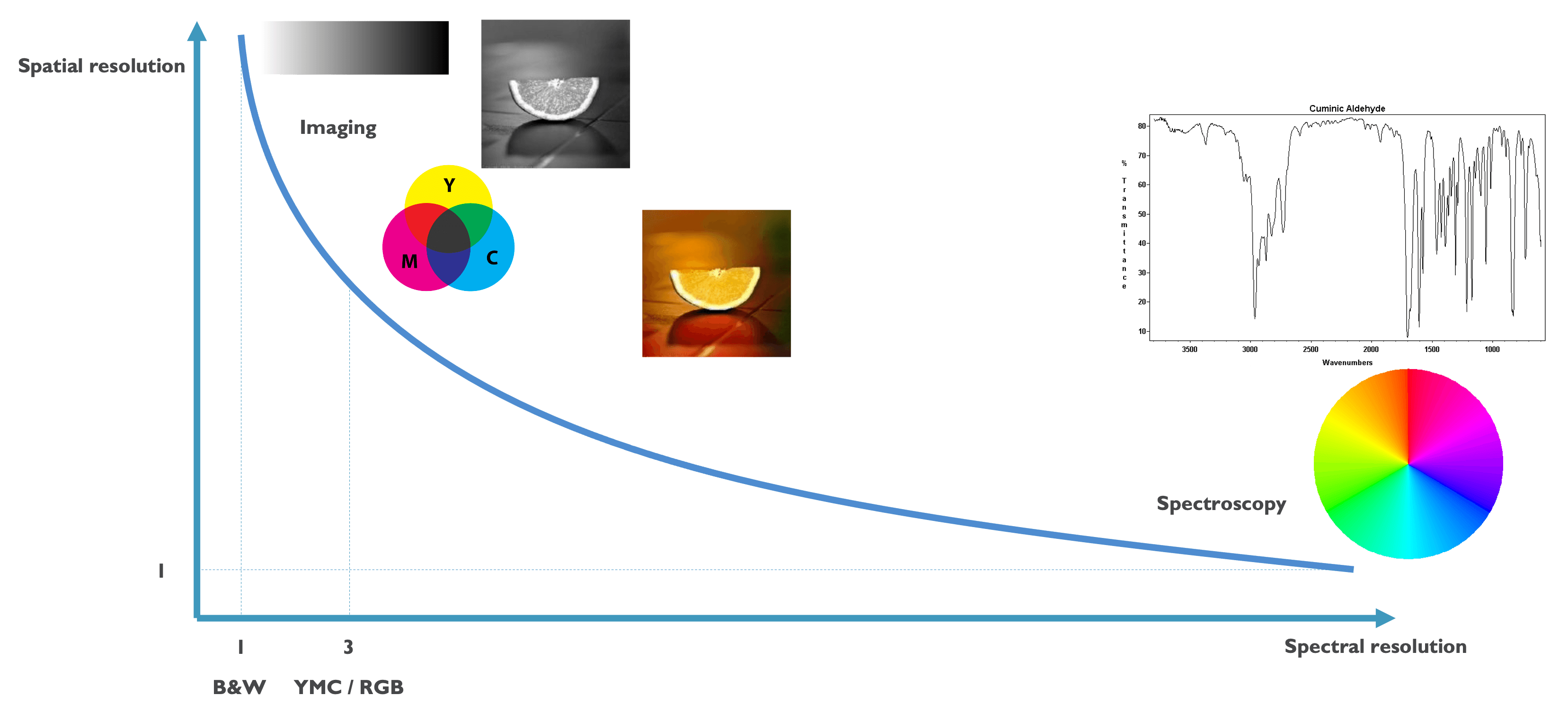
Figure 1 Imaging versus spectroscopy. While imaging explores a larger area in a few spectral components (blue, red, yellow), spectroscopy visualizes the whole spectral signature of one component.
The sound of light
PA combines light and sound to create an image based on the photoacoustic effect first discovered by Alexander Graham Bell over a century ago. Bell noticed that certain materials emit sound when struck by pulses of light. Absorption of the light causes the molecules in those materials to heat up. Heat in turn produces pressure changes when the molecules expand and relax and push against the surrounding tissue. This pressure or sound wave can be detected by a (array of) microphone(s) and reconstructed to a high-resolution image (Figure 2).
“The advantage of PA is that you don’t focus with light –which gets attenuated in the tissue– but with sound. Only the target molecule or structure that absorbs the light will selectively send pressure waves. That means that you can achieve “optical contrast” images at deeper locations and in turbid structures. There is also no need for fluorescent labels or tagging. By tuning the wavelength of the laser beam you can enhance the contrast of target structures or by using different wavelengths you can visualize different structures in one image. An interesting application is the detection of oxygen saturation levels in blood hemoglobin, where oxygenated and deoxygenated hemoglobin absorbs at different wavelengths,” explains Hilde Jans. “These characteristics apply for spectroscopy as well, leading to a technique with zero background and a very low limit of detection. When you shine light onto a sample, it will only send out acoustic waves if the slightest quantity of a particle is present and absorbs the light.”
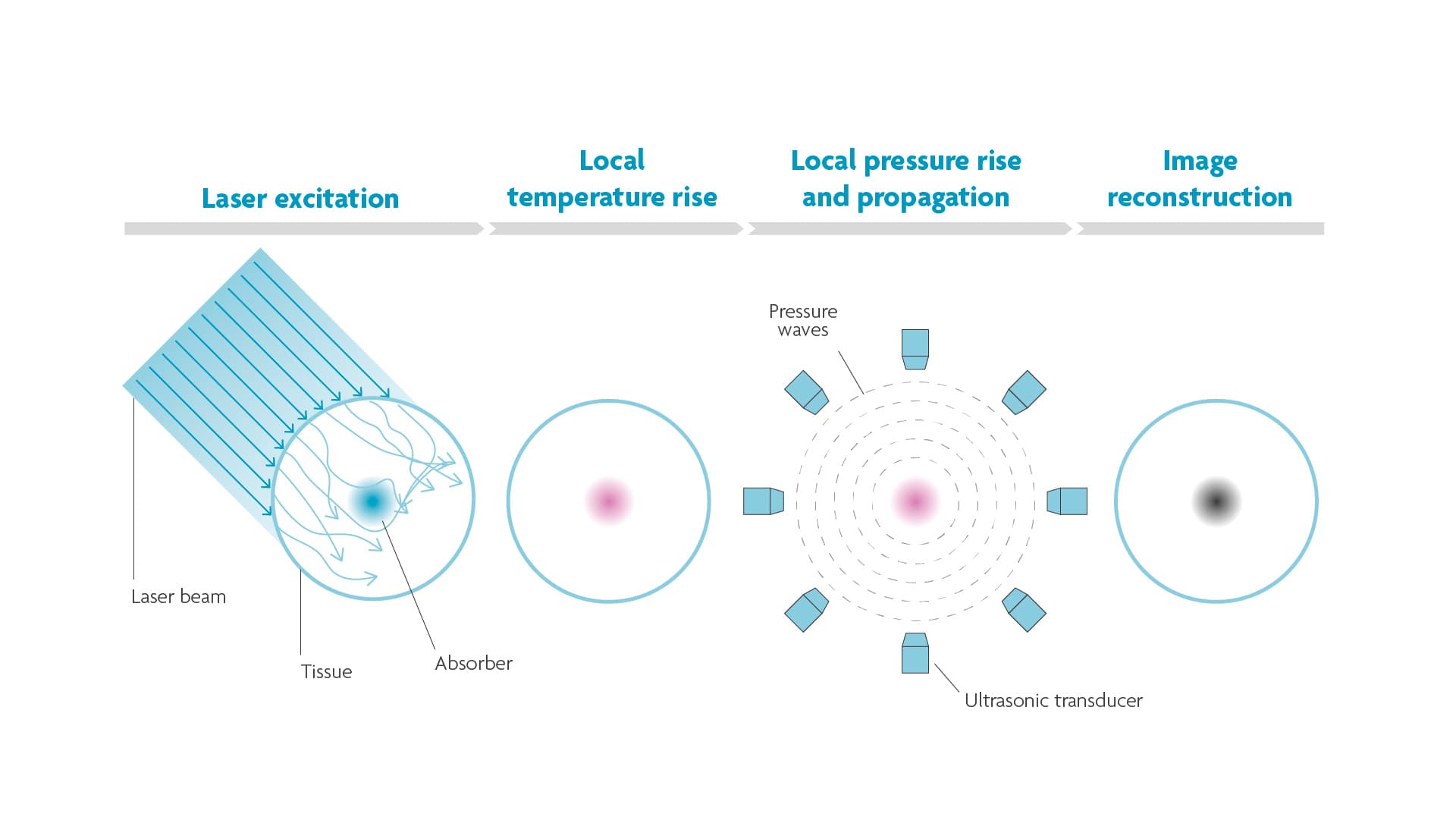
Figure 2. The photoacoustic principle. When pulses of light hit tissue, the molecules that absorb the light will expand and relax due to heat. These vibrations cause a pressure wave that can be detected and reconstructed to an image.
High-sensitivity ultrasound microphones
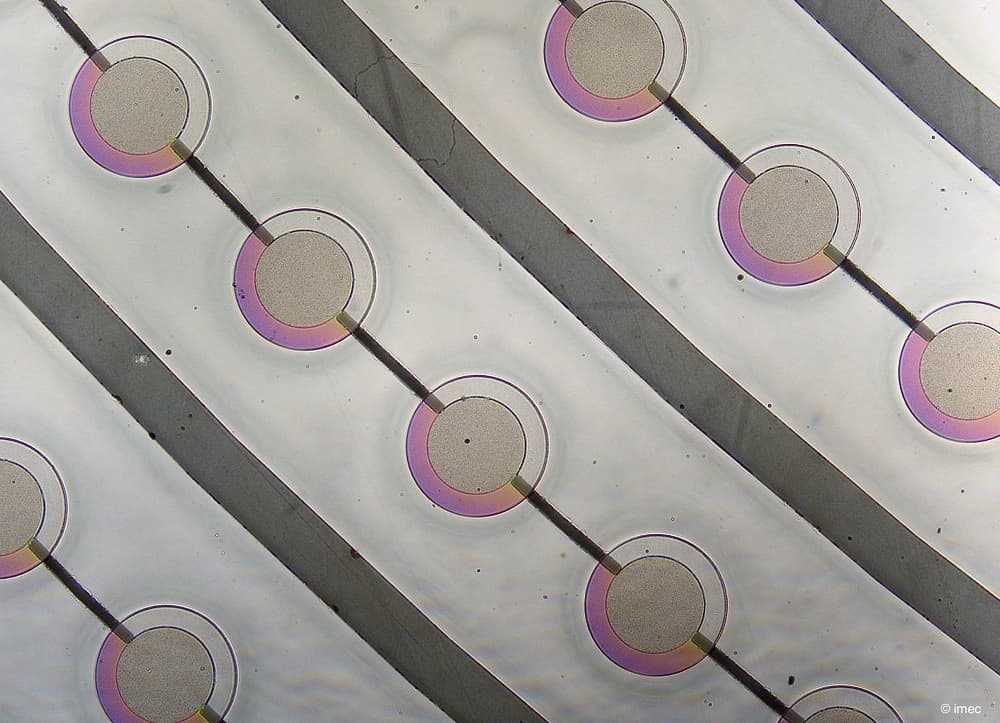
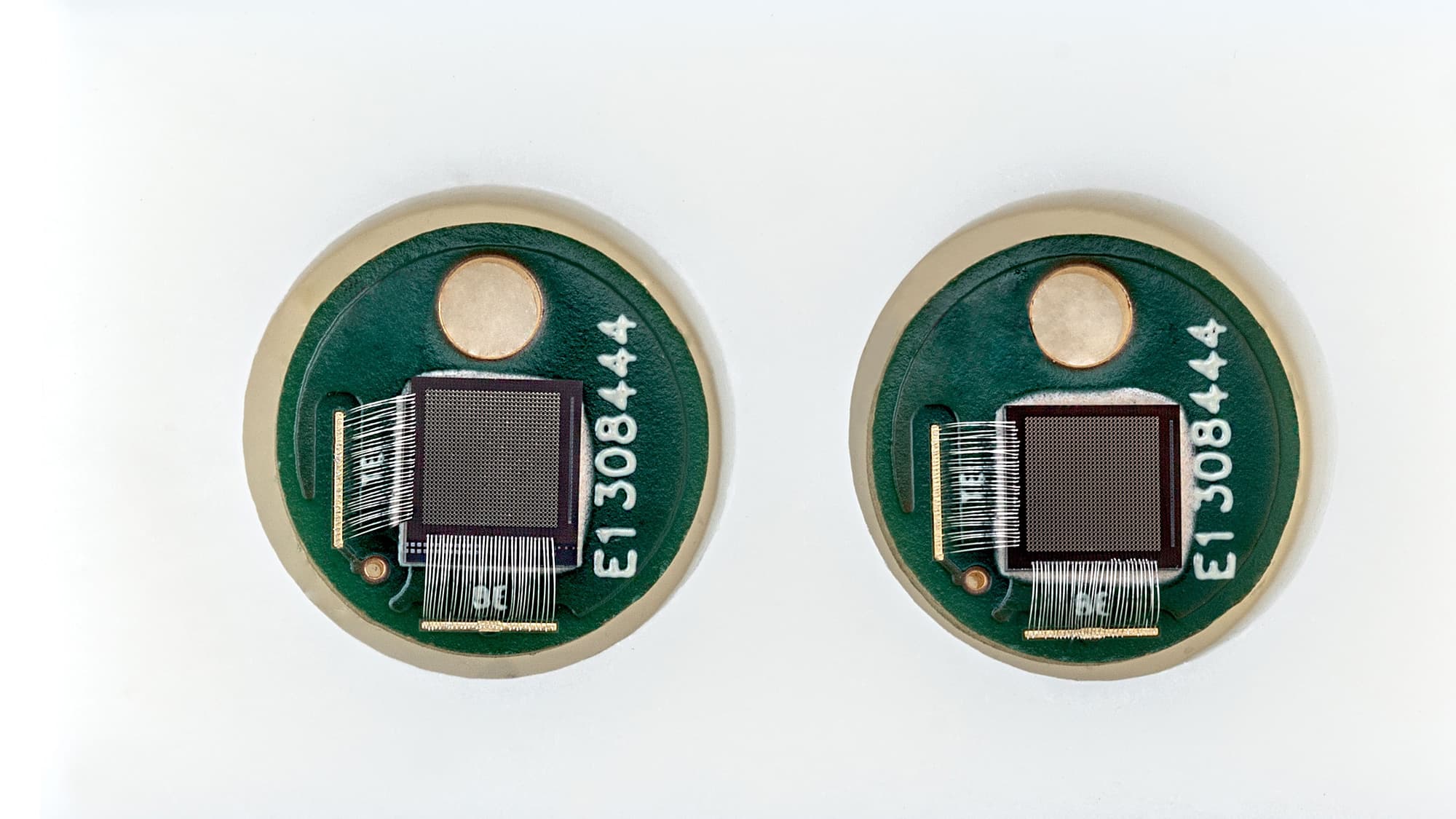
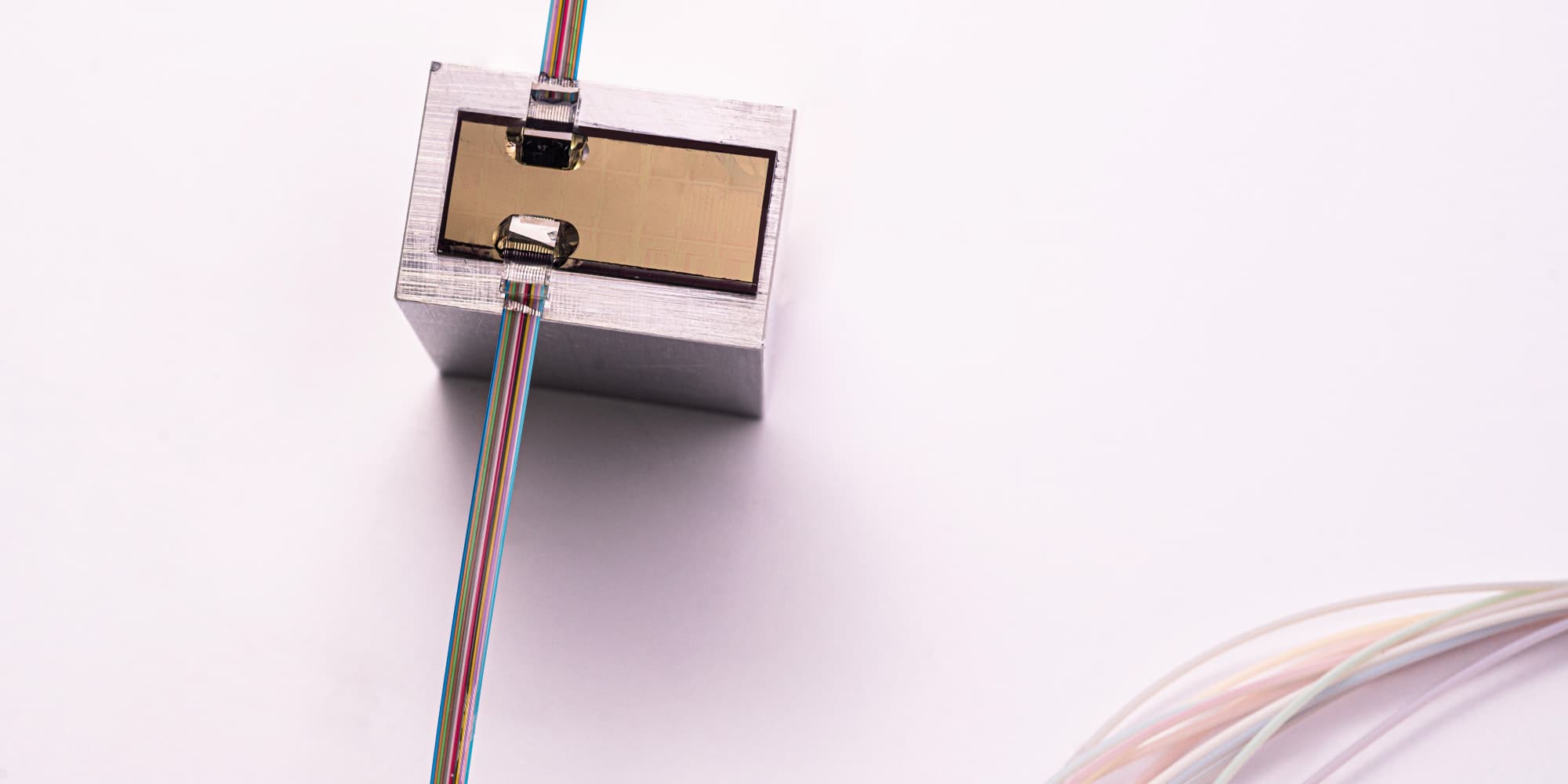
Hilde Jans: “Semiconductor technologies enable the integration of both ultra-sensitive microphones and light sources with high spectral purity onto a chip, bringing photo-acoustic sensing to the next level. On the detector-side, a large, dense array of high-sensitivity ultrasound microphones with high-bandwidth readout is required. The signals in PA are very small because of tissue attenuation. The more sensitive and low noise the microphone is, the deeper in the tissue you can listen. Imec’s current opto-mechanical ultrasound sensor is considered best-in-class for photoacoustic and ultrasonic imaging (Westerveld, 2021). It is based on an opto-mechanical waveguide rather than a piezoelectric crystal to convert sound to a measurable optical signal (Figure 3). The novel approach results in a detection limit two orders of magnitude better than state-of-the-art piezoelectric elements of identical size. This enables applications like through-skull functional brain imaging, where the pressure waves are very small because of the strong ultrasound attenuation of bone. Moreover, a fine-pitched matrix of these tiny sensors can be easily integrated on-chip with photonic multiplexers, opening up the way to new applications such as miniaturized catheters”.
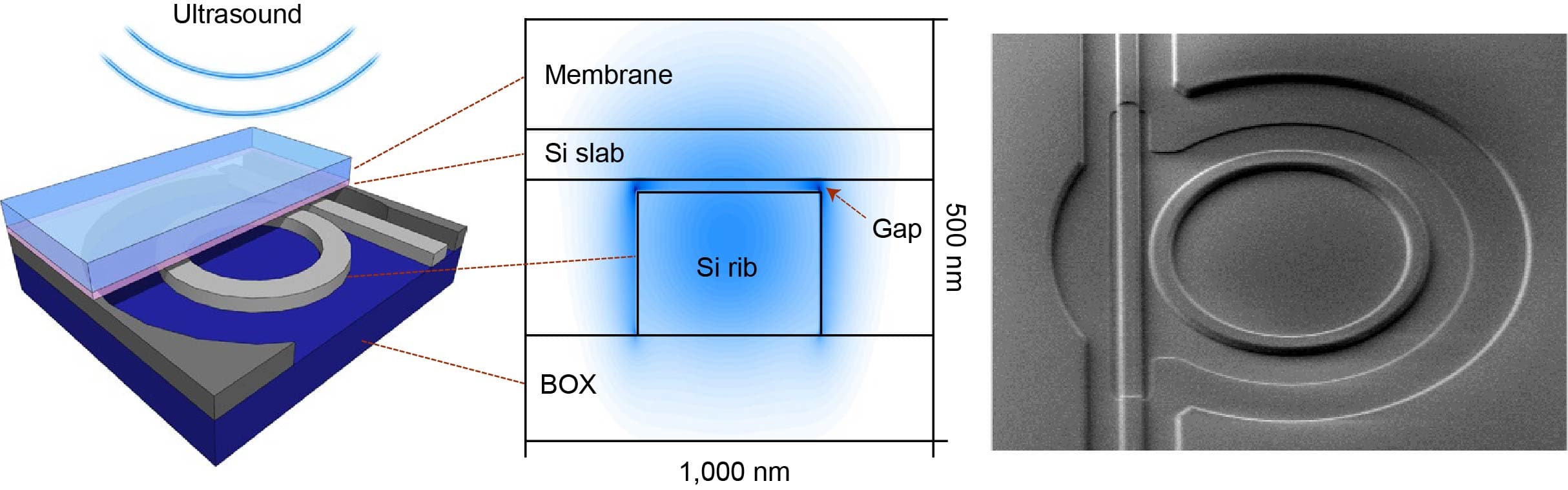
Figure 3. Cross-section and SEM image of imec’s opto-mechanical ultrasound sensor.
Light to create sound
For imaging purposes, light sources typically have one or a few wavelengths. The target structure will absorb light at a preferred wavelength. A second or third wavelength can then be used complementary to create a background for the target structure. Imaging light sources are high-power to guarantee sufficient energy density to generate an image in a large volume of tissue (about 1cm3). Finally, they need to be able to pulsate light. A single thermal expansion of a molecule will not give rise to a pressure wave. For that to happen, the molecule also has to relax back. It is the alternating expansion and relaxation that arises when you pulsate the light, that creates a detectable sound wave.
For spectroscopy, the requirements are a bit different. In this case, you need a tunable light source, or a light source with a broad wavelength range that you can modulate to generate the acoustic signal. However, the technique often requires the acquisition of many separate images at each wavelength of interest, which prolongs imaging time and creates errors when the sample moves between acquisitions. A dual comb laser would constitute an elegant solution for this issue and is therefore under research for photoacoustic applications.
An optical frequency comb simultaneously generates thousands of discrete optical frequency bands that are evenly spaced and very narrow, just like the teeth of a comb (Figure 4). In a dual comb source, two combs are combined, one with the frequencies slightly shifted compared to the other. Pairs of comb teeth, one from each comb, interfere with each other resulting in ‘beating’. The beat notes are detected by the microphone. The average optical frequency of each pair is modulated with a unique acoustic frequency, in other words, the optical absorption spectrum is copied into the acoustic domain. For example, for the ‘green’ wavelength comb pair, the average green will be absorbed by the target molecule and produce a unique tone with a frequency equal to the difference between the two ‘green' optical frequencies. If the microphone picks up a signal at the green acoustic frequency, you can see a spectral peak at that frequency.
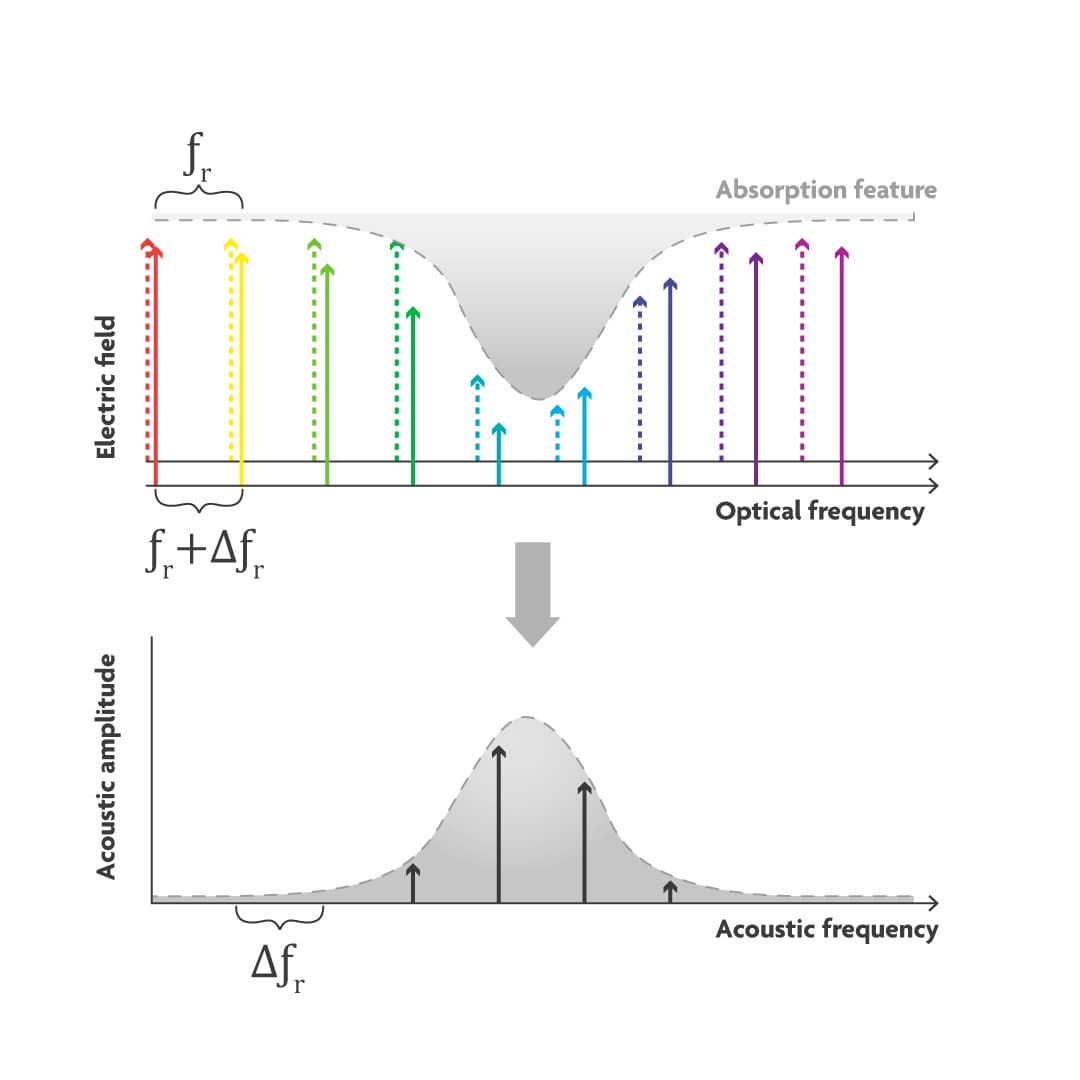
Figure 4 Principle of a dual-comb light source. Two frequency combs with slightly different optical frequency bands interact to generate beating. Microphones can detect the beat notes on a unique frequency when the light is absorbed.
Imec together with the Photonics Research Group, an imec research group at UGhent, recently created a mode-locked laser –the most popular light source to generate a dual comb– that can be integrated on chip (Figure 5) (Hermans, 2021). On-chip integration opens the perspective of miniaturized, stable and low-cost laser sources. Current demonstrations on a silicon platform have shown a limited performance regarding pulse energy, noise and stability due to relatively high waveguide losses and temperature sensitivity of the platform. Imec’s integrated mode-locked laser is fabricated on silicon-nitride (SiN). SiN is one of the main photonic integration platforms that features very low waveguide loss and low temperature sensitivity compared to, for example, silicon. The result is a first step towards high-pulse-energy, low-noise, on-chip mode-locked lasers that imec is researching as a candidate for dual-comb PA spectroscopy.
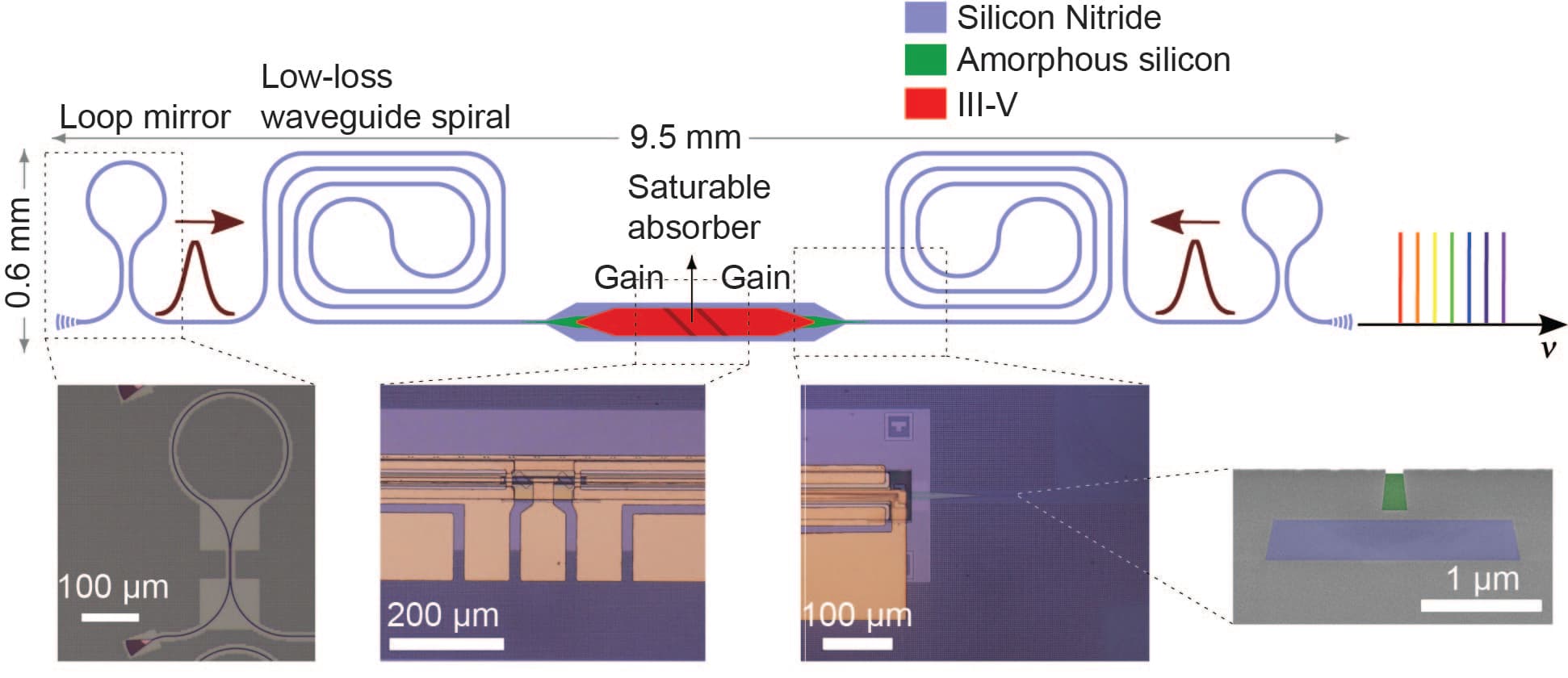
Figure 5 Schematic figure of the mode-locked laser on SiN imec and the Photonics Research Group of imec-UGhent designed.
The spectrum of light sources
“The dual-comb laser is the Rolls Royce of light sources but not all applications require such a fancy light source,” explains Xavier Rottenberg. “CO2, for example, has such a large absorption-peak at 4.3μm wavelength that it can be detected with a simple black body radiator that emits a broadband, continuous spectrum. CO2-sensing is rather an exception; a good spectrometer for complex sensing of similar components still requires a good light source with narrow spectral bandwidth such as arrays of quantum cascade lasers or the dual-comb lasers. Aside from these high-end sources, imec is also working on mid-end light sources based on LEDs (light emitting diodes). Both for imaging and spectroscopy LEDs are interesting candidates because they are low-cost, robust and easy to use. The challenge with LEDs, on the other hand, is that they don’t produce a spectrum right away such as a dual comb laser. By combining two to six LEDs you can already achieve a rough spectrum. Though resolving the absorption peak will be difficult, with correlation of background and other processing techniques it is possible. Current work in imec is focusing on an array of LEDs on-chip in the visible range.”
Application space
PAI and PAS are emerging as new, non-invasive techniques for biomedical applications that fill the gaps between existing modalities (Figure 6). “PAI is particularly well-suited to image blood vessels and oxygen saturation, since haemoglobin (the oxygen-carrying component in red blood cells) has a strong PA signature. Hence, the diagnosis of tumors which often show neovascularization, is a potential application domain for PAI. In particular, PAI is investigated as an alternative for mammography. Mammography is the primary inspection method today for breast cancer. However, it can be painful, involves exposure to X-rays and still shows difficulties to detect tumors in dense breast tissue. PA can reach depths between 5 and 10cm, does not use harmful radiation and can clearly show new networks of vessels around the tumor by tuning the light source to the absorption frequency of haemoglobin,” tells Xavier Rottenberg. Other imaging applications include functional brain imaging, detection of arteriosclerosis and retinal imaging.
“PAS can be employed to detect biomarkers in blood such as cortisol, or for breath analysis. The Holy Grail, however, is non-invasive blood glucose sensing which is essential for diabetes patients. It is a challenging application because the glucose signal is often weak due to the differences in human skin and changes the skin undergoes depending on the environment. Once you would have a robust glucose sensor, you would also be able to learn about glucose metabolism and how the concentration changes because the signal will be stronger when glucose concentration is higher. And most importantly, finger-pricking would be a thing of the past,” concludes Xavier Rottenberg.
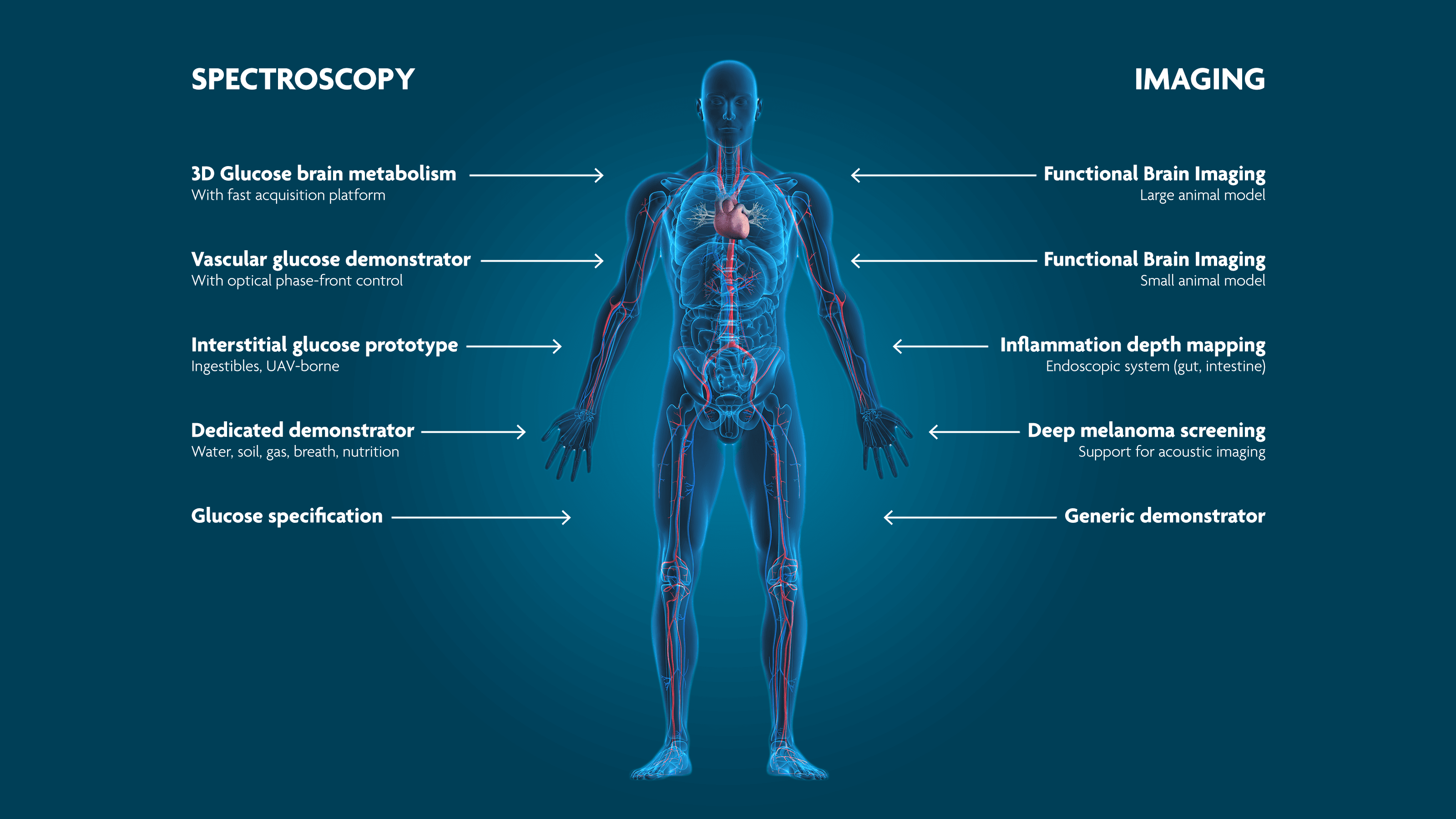
Figure 6 Photoacoustics enables non-invasive, high-resolution sensing for biomedical applications.
Want to know more?
- Read the PR about the OMUS
- Request the papers through our application form
- Hermans et al. High-pulse-energy III-V-on-silicon-nitride mode-locked laser. APL Photonics 6, 096102 (2021).
- Westerveld et al. Sensitive, small, broadband and scalable optomechanical ultrasound sensor in silicon photonics. Nature Photonics, volume 15, pp 341–345 (2021).
- Watch our video on PAI PAS below
- Read our brochure on Photoacoustic Spectral imaging

Dr. Hilde Jans received her PhD in Chemistry from the Catholic University of Leuven in 2010. At this moment, Hilde is a Project Manager in the Bio-photonics team of the Life Science Technologies department at imec. Her main activity is to link the (bio-) application to the technology under development in the team since her expertise is on (bio-)assay development and spectroscopy applications (Raman and photoacoustics). Hilde is involved in many projects, and leading both bilateral and European projects.

Dr. Xavier Rottenberg obtained his MSc degree in Physics Engineering and the DEA in Theoretical Physics from the Université Libre de Bruxelles in 1998 and 1999, respectively. He obtained in 2008 his PhD degree in Electrical Engineering from the KU Leuven. He worked one year at the Royal Meteorological Institute of Belgium in the field of remote sensing from Space. He has been at IMEC Leuven since 2000, where he contributes to research in the field of RF, RF-MEMS, photonics and microsystems modelling/integration. As imec fellow, he currently leads the Wave-based Sensing and Actuation Developments, working among other topics on integrated photonics, flat optics, acoustics, photo-acoustics and M/NEMS. He has (co-)authored over 150 peer reviewed publications, has been granted various patents and co-founded Pulsify Medical in 2019, a young company developing ultrasound imaging patches.
Published on:
15 February 2022