If the Covid-19-pandemic has taught us one thing, it is the importance of fast diagnostic tests. Having these tools at hand helps control the spread of the disease and ensures timely treatment. Molecular diagnostics or DNA-based detection using PCR has become the gold standard for infectious disease testing. For infectious diseases, in particular, its high sensitivity is a major advantage because it enables the detection of lower amounts of disease agents and, therefore, earlier detection of the infection.
Unfortunately, DNA-analysis from extraction, over amplification, to sequencing for disease variants typically involves complex and time-consuming assays with many cycles of adding reagents and washing steps. Ideally, a screening device is small, fast, and easy to use. This is where microfluidics starts to make a significant impact on medical diagnostics. Microfluidics refers to miniaturized chips that can analyze a tiny amount of fluid, such as a drop of blood or even a small fraction of that. Not only does microfluidics reduce the volume of reagents and overall size, but the technology also stands for a whole new way of working. It is often called “lab on a chip” as it performs a variety of lab functions on a single chip. Miniaturizing laboratory workflows with microfluidic technology enables high-throughput diagnostic testing in a matter of minutes instead of hours.
Silicon has been one of the first materials for creating microfluidic structures and offers several advantages. It is very reliable and can be mass-produced. Most importantly, the superior properties of silicon and the highly advanced silicon industry enable extremely accurate and intricate features such as microcavities, microchannels, valves, and pumps. Notably, silicon is still the best choice for shrinking dimensions of microfluidic components and combining them with electrical functionality and sensing modalities.
Chengxun Liu, R&D Team leader for microfluidics technologies, talks about how imec’s silicon microfluidics program accelerates different steps in DNA-based testing procedures, from sample preparation to sequencing.
DNA extraction and capture from liquids
The first step in most DNA-based workflows involves isolating the DNA from a liquid sample matrix, including blood serum, saliva, urine. Solid-phase extraction, most often the “Boom method”, is the de facto standard for capturing DNA such as the whole genome or circulating cell-free DNA fragments from complex liquids. The Boom method has two steps. First, DNA is absorbed on the surface of micro beads or pillars while other molecules pass through. Afterward, DNA is washed off and collected for downstream analysis. The downside of this approach is that it is labor-intensive, difficult to control, requires complex fluidics, and has low efficiency because it’s hard to be sure that you’ve eluted all DNA from the beads or pillars in the end. This is especially an issue for early disease detection where the target DNA is often extremely low in quantity.
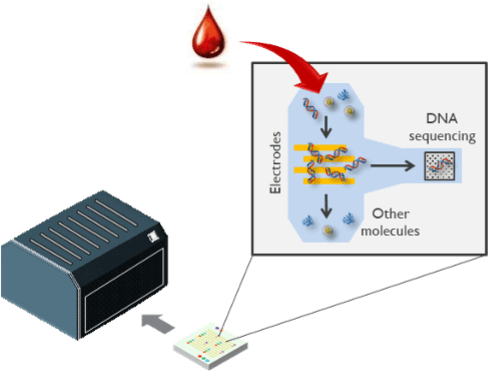
A schematic of integrated electrical DNA extraction and sequencing. When the electric field is switched on, the DNA molecules are ‘caught’ and the medium can be removed. The molecules can be released into a new solution when the electric field is switched off.
To answer the time-consuming workflow of sample preparation, Chengxun Liu’s team investigated using electric fields to extract DNA from a sample. In the dielectrophoresis (DEP) technique, an electric field is applied between micro-electrodes which causes small molecules such as DNA to be attracted to the electrodes. This electrostatic attraction is similar to lifting a piece of paper by a comb after running the comb through your hair, but the effect is more pronounced on microscales. Switching on the electric field captures the DNA on the electrodes and allows for washing off the excess fluids. Switching off the electric field releases the purified DNA and makes the molecules ready for downstream analysis. DEP simplifies DNA extraction from the complex multi-step fluidic operation to simply turning on and off the electric field. Consequentially, this approach can be controllable, automated, and miniaturized. Researchers have already demonstrated electrical DNA extraction in complex mediums such as serum.
DNA capture from aerosols
A novel modality which was recently added to the microfluidic library is the possibility to capture aerosol particles that could contain molecules indicative of infectious diseases.
This building block opens up possibilities for viral particle detection in ventilation systems as well as breath analysis, and this not only for COVID-19 but for any infectious disease that spreads via aerosols.
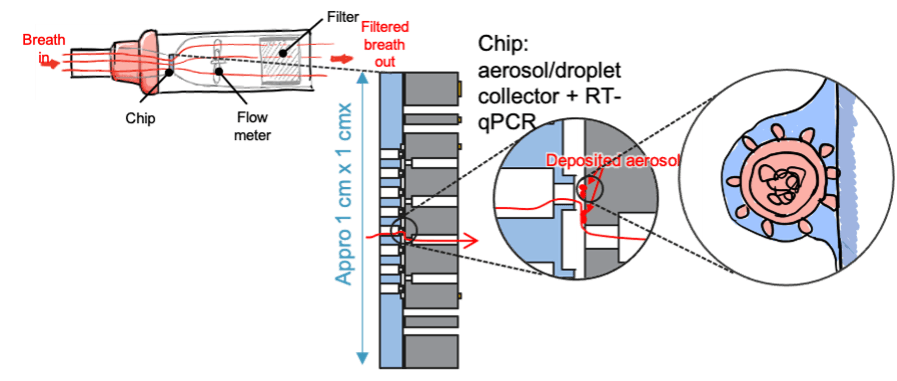
Microfluidic chip for DNA capture fro aerosols e.g., in a device for breath analysis.
DNA amplification integrated with dielectrophoresis extraction
The diagnosis of infectious diseases is based on the presence or absence of genetic material from micro-organisms. Because of the minute amounts of target DNA, detection goes hand in hand with amplification which is typically performed through a so-called polymerase chain reaction (PCR). The importance of PCR for any DNA-related analysis has led to the development of several microfluidic devices and process flows. For example, imec has demonstrated an ultra-fast microfluidics chip for PCR that brings the amplification time for 40 cycles down to 3 minutes, which is 20 times faster than in a conventional protocol. For a quantitative PCR, you receive the results immediately
Although a PCR process enables very sensitive DNA detection, it is vulnerable to contaminants. Not only the target DNA molecules are exponentially duplicated, but also contaminant DNA. Therefore, simple, controllable, and clean DNA extraction is crucial to minimize contamination sources for correct PCR results. In this sense, electrical DNA extraction with dielectrophoresis offers the added advantage that it can be easily combined with PCR on the same chip. While DNA is electrically captured and retained, assay fluid can be loaded into the chip, and the next reaction can be started. That means that the chip can accommodate a series of extraction-reaction steps in a single fluidic channel, adding reagents and removing waste. Such flexibility is crucial when different sample preparation workflows are required for different applications, e.g. for other genomic applications or other sample characteristics.
While PCR is sometimes the end point, there are a number of genomic workflows that use PCR as an intermediate step. One of the critical challenges of sequencing DNA is that the amount of DNA from the raw sample is often orders of magnitude lower than what can be measured by a (not so sensitive) DNA sequencer. Therefore, the purified DNA must first undergo whole genome amplification (WGA) to generate sufficient DNA copies. Dielectrophoresis can help in this process, because the assay fluids are substituted while DNA is held in situ the assay reaction volume can be maintained relatively constant throughout the protocol. This is an advantage over typical WGA workflows where volumes expand as reagents are subsequentially added in each step. Therefore, electrical DNA capture enables high-throughput DNA processing, for example, for whole genome analysis for thousands of single cells.
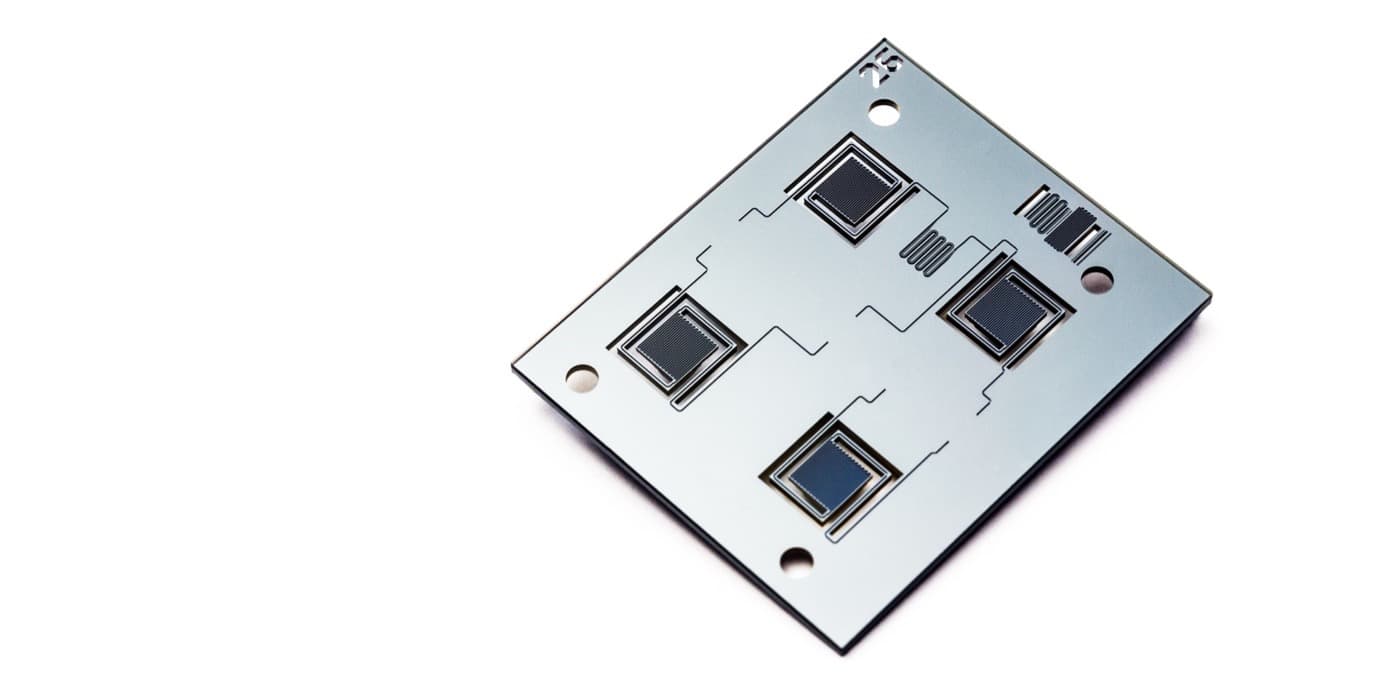
Imec’s ultra-fast PCR chip that goes from sample to result in minutes.
Ultra-fast, high-throughput sequencing
The most informative way of reading DNA or RNA information is sequencing. Sequencing by synthesis is the de facto standard accounting for 90 percent of the world’s sequencing tests. By sequentially adding fluorescently-labeled DNA-building blocks (oligonucleotides A, G, T, C) the complementary strand of the target DNA is synthetized. The fluorescence can be detected after each cycle, and like that, the whole DNA-sequence is read out.
One of the problems with sequencing-by-synthesis is that it is extremely time-consuming. The four oligonucleotides must be added in each cycle with a washing step in between. Since sequencing requires more than 1,000 cycles, you end up with over 800,000 steps. By creating a small and uniform microfluidics system, we can gain time on sequencing because the washing steps are substantially shorter and more efficient. With the micro-cavity chip, the volume is minimal and can be replaced several times per second. It would improve throughput, efficiency and reduce costs for sequencing applications.
Imec devised a method to bring those reagents to the microcavities much faster then in conventional fluidic systems. The chip consists of high-density individual micro-cavities connected with microfluidic channels to supply reagents e.g., the four oligonucleotides and washing agents. There are currently over millions of micro-cavities on one chip. The chip makes excellent use of silicon technology to create fine fluidic features with submicrometer resolution. The smaller the features, the more micro-cavities we can fit into a unit area, and the higher the sequencing throughput we can achieve. Using silicon also means that we can easily make precise structures for both chamber and channels connecting all micro-cavities. Consequently, we can ensure that all micro-cavities get flushed or switched from one liquid to another very fast and equally.
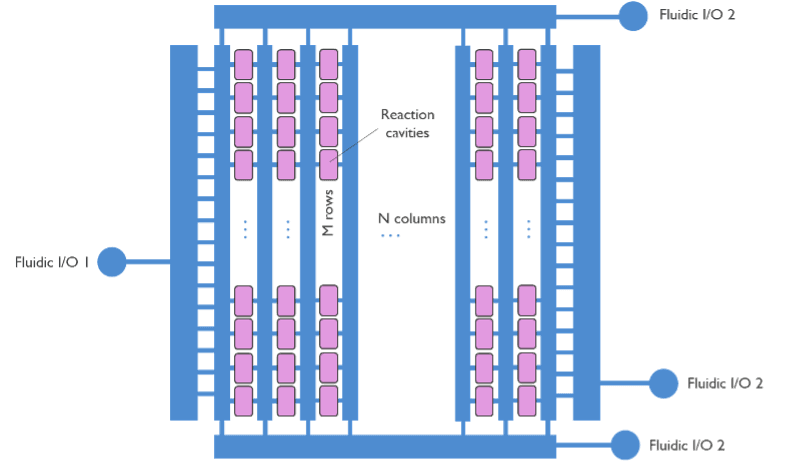
An array of fastfilling micro-cavities on a chip with 2 inlets and an outlet.
From microfluidic chips to fully-integrated diagnostic tests
Over the years, imec has developed microfluidic building blocks for different DNA-related analysis that can be tailored to the application at hand. Still, the microfluidics need to be coupled to become a whole system. Today, when you want to operate a small chip, you usually still need bulky equipment around it, such as external pumps and valves, that have an intrinsic mismatch in volume compared to the chip format. That’s why current activities at imec also focus on a more sophisticated on-chip fluidic handling to control the fluidic actuation. This effort includes integrated micro-pumps and valves, heterogeneous silicon-glass-polymer integration platforms, and massive micro-droplet handling with in-flow sorting or electrowetting.
Having a small testing device at the point of need that renders results in a matter of minutes will be a gamechanger for infectious disease detection. The availability of such a rapid test, e.g., in hospitals, enables screening for contagious patients or staff at any moment.

(Right) Today’s microfluidics (left) versus a future vision of fluidics integrated on-chip.
This article was previously published in Nikkei BP (https://xtech.nikkei.com/atcl/nxt/column/18/01638/043000001/)
Want to know more?
Read the webpage on microfluidics.
Download our white paper on semiconductor technologies and system concepts to revolutionize genomics.

Dr. Chengxun Liu is currently a program manager and principal member of technical staff in the life science department at imec. His main responsibility is to identify and develop microfluidics solutions for life science applications. Chengxun has a mixed background of engineering and biology, with the belief that microtechnology enables innovative solutions to life science challenges. His research interest and experience covers a broad range of biophysics and microfluidic technologies, such as cell isolation, molecular assays, electrokinetics and droplet fluidics. Being a principal scientistn Chengxun has been leading the development of several technologies such as single-cell electrical impedance spectroscopy, high-speed cell sorting, droplet sorting & programmable processor, electrical cell & biomolecule manipulations, etc.
Published on:
21 May 2021