1. What market does BioTelemetry target?
Joe Capper, President and CEO of BioTelemetry: "Our goal is to help people with heart problems, such as arrhythmia. With arrhythmia, the heartbeat is irregular – too fast or too slow – and, in some cases, even causes sudden death. Around 20-30% of strokes in the U.S. are caused by arrhythmia."
"In the past, these patients would typically wear a Holter monitor, prescribed by their physician, for 24 or 48 hours. A Holter device is like a body-worn ECG monitor, and it is typically worn on one’s belt with wires attached to the chest and abdomen."
"After wearing the Holter, patients had to deliver it back to the hospital, where experts downloaded the information and ran it through signal processing software to interpret the ECG signals and detect abnormalities. It was a rather slow and time-consuming process."
"The Holter was a huge step forward from ECG readings at a hospital, but it still only covered a limited period of time. Arrhythmia can be intermittent: going away for a couple days, and then coming back again. There was no guarantee that a patient would have an arrhythmia event during the 24/48h measuring period."
"BioTelemetry was established in 1994 to change the lives of arrhythmia patients. We wanted to create a more patient-friendly and smarter device that could even be used to alert patients and physicians when a situation became life-threatening."
2. What product & service does BioTelemetry offer?
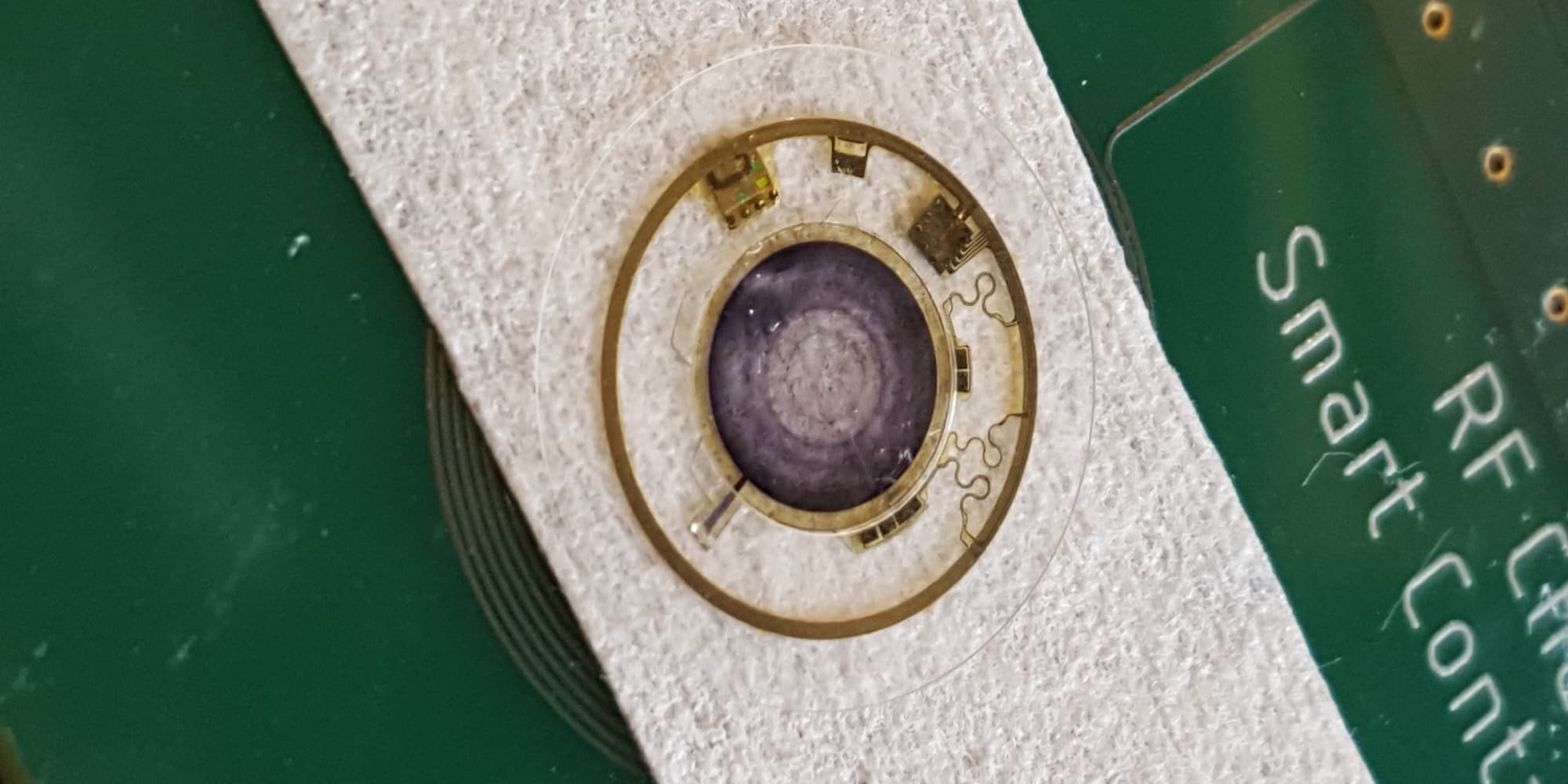
Joe Capper: "Our flagship device is the MCOTTM which stands for 'Mobile Cardiac Outpatient Telemetry'. It is a patch with ECG recording technology and specific algorithms embedded to recognize arrhythmia events. Dedicated radio chips ensure communication with the wearer’s mobile phone."
"Data is transmitted, in near-real time, to a monitoring center with certified cardiac technicians. The technicians screen the data and, when the situation might be life-threatening, alert a physician."
"The results are impressive. The monitoring period now extends from 2 days (with the standard Holter monitor) to 30 days (with the MCOT), and the diagnoses increases from 15 to 90%. This is a huge improvement from a healthcare perspective."
"Typically, a physician prescribes our patch and service, and our team provides the device to the patient and analyzes the data. We send a report to the physician or alert the physician if there are alarming signs. The insurance company usually pays for the service."

BioTelemetry's flagship device is a patch with ECG recording technology and specific algorithms embedded to recognize arrhythmia events. Dedicated radio chips ensure communication with the wearer’s mobile phone.
3. Your company is on Fortune Magazine’s 100 Fastest-Growing Companies list for 2019. What’s the secret to your success?
Joe Capper: "That’s simple: a good team, and a sound and flexible strategy. One of the cornerstones of every successful company is building a strong team. Finding and recruiting the right talent certainly takes time, and it’s worth the effort."
"We recently hired a new CTO who has tremendous experience in the field of big data, algorithm development, etc. Incorporating new expertise in the company, matching core competencies and addressing market needs is essential to staying ahead of competitors and making the company future-proof."
"Once a strategy is in place, it’s really about executing it in a very disciplined way. We favor proven, old-fashioned principles like maintaining a healthy balance sheet and a positive cash flow. We invest in tech innovation. In our opinion, these are sound principles that are also very common sense."
4. What’s your vision on the future of healthcare?
Joe Capper: "For some years now, we have been experiencing a – very slow – shift toward increased remote monitoring and telehealth services. There are two reasons for this, first, to reduce the cost of care, and second, to address the shortage of healthcare providers."
"Our MCOT device fits into this trend as it allows patient monitoring for 30 days in real-life conditions and in near-real time. Previously, the only way to get this amount and quality of data was to hospitalize patients and restrict them to telemetry beds, which was extremely expensive."
"Compared to other industries such as retail, manufacturing or automation, the healthcare industry is very slow to adopt new technologies that drive down cost. Combine this with an aging population, and the pressure on the system builds up enormously."
"The demand is there, and the connected health technologies are available, so there will be a natural shift toward increased remote monitoring and telehealth services."
5. Is there an impact of the COVID-19 pandemic on the adoption of new technologies in healthcare?
Joe Capper: "As I shared, the healthcare industry is very slow to adopt changes. COVID-19 might be the extra push that is needed to make new technologies find their way into the daily practice of physicians and hospitals. The emphasis is now on social distancing and staying at home, and this will probably act as an accelerator for a faster transition toward telemedicine and remote monitoring."
"In the U.S., many physicians were forced to implement the use of telehealth overnight and saw a tremendous increase in online visits during the first two weeks of April—up to a 1000%! They were not initially prepared for this shift, but they are succeeding in making this new reality work."
"We also experienced certain telehealth restrictions being relaxed, making it easier to implement a new telehealth model. For example, there are certain rules that prevent providing healthcare across U.S. state lines. These restrictions have been relaxed to allow more telehealth in the fight against COVID-19. This has been an enormous advantage because people can access care much faster than when these restrictions were still in place."
"Additionally, insurance companies caught up and made the necessary changes so physicians can get paid for telehealth visits. In the past, lack of payment for telehealth visits was an important incentive for physicians to continue examining patients only in their offices and clinics. As a result of relaxed regulation and an improved payment model, telehealth has become a much more attractive alternative."
"I strongly believe that patients don’t want to go back to the old model once the pandemic has passed, and physicians will need to adapt. In many ways, telehealth might be a better way to offer some types of healthcare. The amount of time and money being saved is enormous. COVID-19 is serving as an accelerator to this transition, and physicians need help monitoring, detecting, analyzing and interpreting data remotely. For the analysis and interpretation part, algorithms will play a major role."
6. In 2002, your first MCOT device was approved by the FDA. Why did you turn to imec in 2013 to develop a next-generation device?
Joe Capper: "BioTelemetry was founded in 1994, and in the early 2000s, we received our first FDA approval for our MCOT device. We were very proud to commercialize our innovative technology for remote arrhythmia detection. In 2008, our company went public and raised a lot of money from investors."
"In 2009, Medicare (the U.S. federal health insurance) and private insurances cut reimbursement for our MCOT device by about 35%. This was a tremendous setback and revenues began to decline."
"I joined the company a year later to lead the team through this difficult situation. The first thing we did was reestablish our technology by better communicating its benefits. The evidence was there, so it wasn’t really that hard to do."
"Next, we needed to manage costs to make the company financially healthy again, and we needed to do this while also staying ahead of our competitors."
"It didn’t make sense anymore to develop all of the technology in-house, given the (small) size of our company. We had a lot of expertise in software and signal processing, but in terms of chip technology and hardware development, it was hard to stay up-to-speed with fast-evolving new trends. That is why imec fit nicely as a solution."
"We knew imec had a good reputation as a technology leader but we hadn’t worked with them. We discussed with them how to make our device more patient-friendly and smaller, with a wireless and more flexible form factor – in the shape of a patch – and how to make the technology faster. Our collaboration with imec was the perfect marriage at the perfect time. It was certainly one of our best decisions, and today we continue to have a very good relationship."
7. What specifically did imec develop in this project?
Joe Capper: "Imec and BioTelemetry completed a 2-year development-on-demand project including ASIC and system development, hardware and software design, compliance to regulatory standards, etc. The ASIC design was transferred to a semiconductor vendor that commercialized the chip and volume-produced it."
"It was actually a project that involved three companies: imec developed the electronics; DELTA, a company from Denmark, the patch form factor; and BioTelemetry the smartphone and cloud application."
8. Did imec also assist in the FDA procedure?
Joe Capper: "Yes. Imec provided all of the technical documentation to validate that the device was designed according to medical regulations. Additionally, imec assisted in testing the device with third-party test houses. They provided proof of compliance for hard- and software."
9. Why would you recommend imec as a preferred technology partner?
Joe Capper: "From the beginning, the BioTelemetry and imec teams had a very open way of communicating and were fully aligned. Good communication is key to every successful long-term partnership, whether it’s a business collaboration or a marriage. The imec colleagues are very honest and trustworthy. If they said they would deliver something at a specific time, they did. And when something couldn’t be done in a certain timeframe, they told us ahead of time and elaborated on the reason for it. I would say we have had a good ‘cultural fit’ which made the collaboration work perfectly."
"Also, from a technological perspective, imec is an excellent partner. It’s difficult and costly for companies like ours to reach imec's specific level of expertise. If we can leverage that, it’s wonderful."
10. Are there other benefits – apart from technology development – of working with imec?
Joe Capper: "Thanks to imec, we have met new contacts in the world of technology. This is very valuable. It’s easy to focus on running the business without making time to meet new people."
"I spoke at the imec technology forum in Belgium (ITF, now FutureSummits), and through that experience, I met a lot of new people."
Want to know more?
- Would you like to discuss your idea or collaborate with us? Please contact Zohaib Gulzar, Business Development Manager at imec.
- Take a look at our collaboration models.
- Get a nice overview of imec’s connected health solutions.
- Learn more about imec.
BioTelemetry, Inc. is a leading remote medical technology company focused on the delivery of health information to improve quality of life and reduce cost of care. The company provides remote cardiac monitoring, centralized core laboratory services for clinical trials, remote blood glucose monitoring and original equipment manufacturing that serves both healthcare and clinical research customers. Find more information at https://www.gobio.com/.
In December 2020, BioTelemetry was acquired by Philips. More info: https://www.gobio.com/news/philips-to-acquire-biotelemetry/
Published on:
21 September 2020